Electrons Are Blank in an Ionic Bond
An atom is most stable when there are 8 electrons in the outer layer but most elements do not have this. A polar covalent bond is formed by an.

Chemical Principles Chemistry Lessons Chemistry Education Ionic Bonding
16 Metals tend to _____ electrons and nonmetals tend to ____ electrons to give them full valence shells like a noble gas.
.PNG)
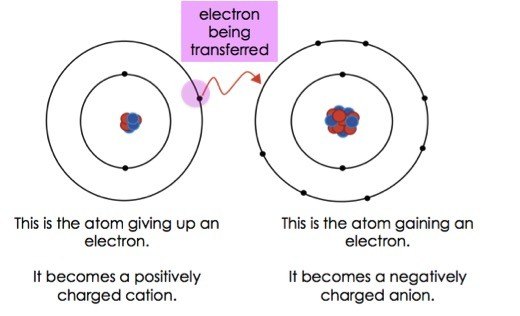
. Loses valence electrons and becomes positive. The oppositely billed ions are drew in per various other by electrostatic pressures which are the basis of the ionic bond. Click hereto get an answer to your question Fill in the blanksAn ionic bond is formed by.
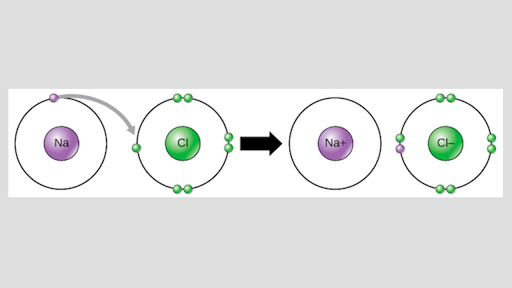
Lets use the ions Cl- and H forming HCl hydrochloric acid chlorine has 7 valence electrons and hydrogen has one so when they form an ionic bond the valence shell is filled. If electrons are transfered completely it is due to very high difference in electronegativity of the com. An ionic bond between atoms forms when the electrons in the outer layers of two different atoms will add up to 8 so they can combine to make a.
Metal atom in ionic bonding BLANKS. This exchange results in a more stable noble gas electronic configuration for both atoms involved. If you want to create an electrical current which situation would produce a solution capable of this.
An ionic bond forms when blank electrons An ionic bond forms when blank electrons Answers. Option D is the correct answer. Nonmetal atom in ionic bonding BLANKS.
Added an answer on December 17 2020 at 536 pm. Electrons are in an ionic bond whereas they are in a polar covalent bond and in a nonpolar covalent bond Chemistry UNIT 2. How many electrons are needed in the outer.
This type of ionic compound is formed when one kind of metal reacts with one kind of nonmetal. 7 Fill in the blanks. An ionic bond is based on attractive electrostatic forces between two ions of opposite charge.
A chemical bond is formed by either sharing or transfer of electrons. Ionic bonds form between metals and ____. The atom that loses the electron becomes a positively.
A covalent bond is formed when. They do NOT conduct electricity in the BLANK form but conducts electricity in the molten state and the aqueous state. Fill in the blanks.
Sulfur S will ____ valence electrons when forming an ionic bond. Properties of Ionic Compounds. Gains valence electrons and becomes negative.
Ionic bonds happen because of the ____ of valence electrons. When atoms join together to form chemical bonds without transferring any electrons but rather by sharing them they are said to be united by a covalent bond. Which of the following does not have a chemical bond that is covalent.
They are typically soluble in water some have very low solubility. Chemistry 21062019 2230 haileywebb8. Ionic bonds are formed when atoms _____ electrons while covalent bonds are formed when atoms _____ electrons.
This difference causes an unequal sharing of electrons such that one atom completely loses one or more electrons and the other atom gains one or more electrons such as in the creation of an ionic bond between a metal atom sodium and a nonmetal. A blank forms between 2 or more non metals. In the compound aluminum oxide which is the cation.
An ionic bond involved the transfer of electrons from one atom to another. Transfer of electrons happens to electrons in an ionic bond. A blank forms between a metal and a non metal or polyatomic ion.
Boron B will ____ valence electrons when forming an ionic bond. Ionic bonds which involve the transfer of electrons occur when the difference in electronegativity between two elements exceeds _____ on the Pauling scale. In ionic bonding electrons are entirely transferred from one atom to an additional In the procedure of either shedding or acquiring adversely billed electrons the responding atoms create ions.
Ionic bonds are formed when atoms transfer electrons. Electrons are transfered in an ionic bond whereas they are unequally shared in a polar covalent bond whereas they are equally shared in a nonpolar covalent bond Step-by-step explanation Ionic bond involves complete transfer of valence electrons between atoms and generates two oppositey charged ions. For instance NaCl is formed by a transfer of one electron from sodium to chlorine.
Electrons are transferred in an ionic bond whereas they are unequally shared in a polar covalent bond are equally blank in a nonpolar covalent bond. Ionic bonds are also formed when there is a large electronegativity difference between two atoms. The electrostatic attraction that exists between ions that were formed as a result of a transfer of electrons.
1 Get Other questions on the subject. For an ionic bond to be possible and energetically favorable the two atoms involved must be blank. The attraction of an atom for a shared pair of electrons.
In contrast covalent bonds are formed when atoms share electrons Theres a distinction between the two. Electrons are in an ionic bond whereas they are in a polar covalent bond and in a nonpolar covalent bond Chemistry UNIT 25. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
Anatomy and Physiology questions and answers. View the full answer. Ionic bonding is BLANK of valence electrons.
When two atoms react to form an ionic bond one atom would completely lose one electron while the other would completely gain that electron. For each blank write a word that is an antonym of the italicized. Ionic bonding forms BLANK.
Atoms in ionic bonding follow BLANK. He has been accused of theft but we feel sure that after the trial he will be Absolved.
Ionic Bonding Biology Definition Role Expii

Introduction To Chemical Bonding Chemical Bond Ionic Bonding Covalent Bonding

Ionic Bond Doodle Graphic Ionic Bonding Organic Chemistry Study Stem Students

Ionic Bonding Periodic Table Quiz Quizizz
.PNG)
Ionic Bonding Presentation Chemistry
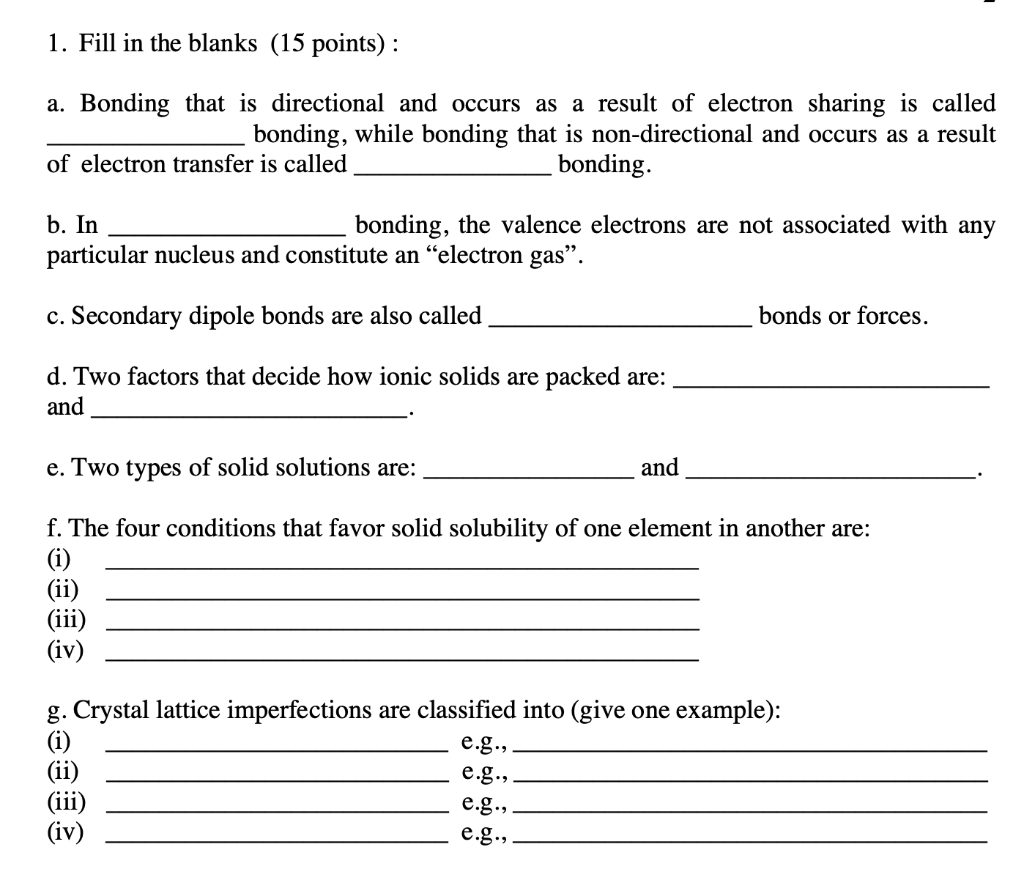
Solved 1 Fill In The Blanks 15 Points A Bonding That Chegg Com
Chemical Bonds Chemistry Of Life Biology Article Khan Academy

Ionic Bond Examples Biology Dictionary

Ionic Bonding Biology Definition Role Expii

Sharanya Saxena Copy Of Ionic Bonds Se Name Sharanya Saxena Date 16 October 2020 Student Studocu

Ionic Bonding Electron Affinity Covalent Bonding

Ionic Bonding Gizmo Answers Name Ashley Maddison Date 11 28 Student Exploration Ionic Bonds Studocu

Homework 1 Purple Due Monday 4 15 Green

Solved Is Denatured And No Longer Functional Bis Denatured Chegg Com

Ionic Bond Examples Biology Dictionary

Solved I Multiple Choices For Each Of The Following Write Chegg Com

Comments
Post a Comment